Case Study
Human-Centered Hygiene: Improving Compliance and Patient Experience Through Design
The Challenge
The COVID-19 pandemic put the spotlight on infection control like never before. Health systems collected massive volumes of surveillance data, revealing critical patterns in disease transmission, patient outcomes, and operational performance.
Landi was tasked with translating these epidemiological insights into real-world solutions—tools that could close the loop between data-driven research and practical intervention.
Our initial investigation focused on the overlap between patient satisfaction and disease transmission. What we discovered was both sobering and actionable:
One of the greatest contributors to both viral spread and poor patient experience was a persistent, system-wide failure to comply with hand hygiene protocols.
Key Findings:
In large private facilities, doctors washed their hands only once for every 3 patient interactions.
In large public facilities, this dropped to once every 42 interactions.
Previous interventions—like signage, audits, and dispensers—had not moved the needle. Our goal became clear:
Create a feedback system that works within the chaos of clinical care, not against it.
Services Rendered
Research and Development
User interviews (ie. comprehensive personas)
Process mapping (ie. end to end user journeys)
User-driven product requirements
Competitor product analysis
Design and Creation
Prototyping Software (low fidelity) (eg. Balsamiq)
Hand-drawn industrial designs
Photoshop renderings (ie. customer feedback)
Solidworks with PLM (ie. Complete rendering and creation)
Software Development
Platform Architecture (eg. AWS loT)
Application Development (eg. React, Python, Cognito)
Embedded Linux (eg. device operation)
Automated Testing and Build (eg. Github Actions)
Project Management
Modified Agile Hardware Design (MAHD)
JIRA CMMI for internal work tracking
Harvest Integration for project budgeting and oversight
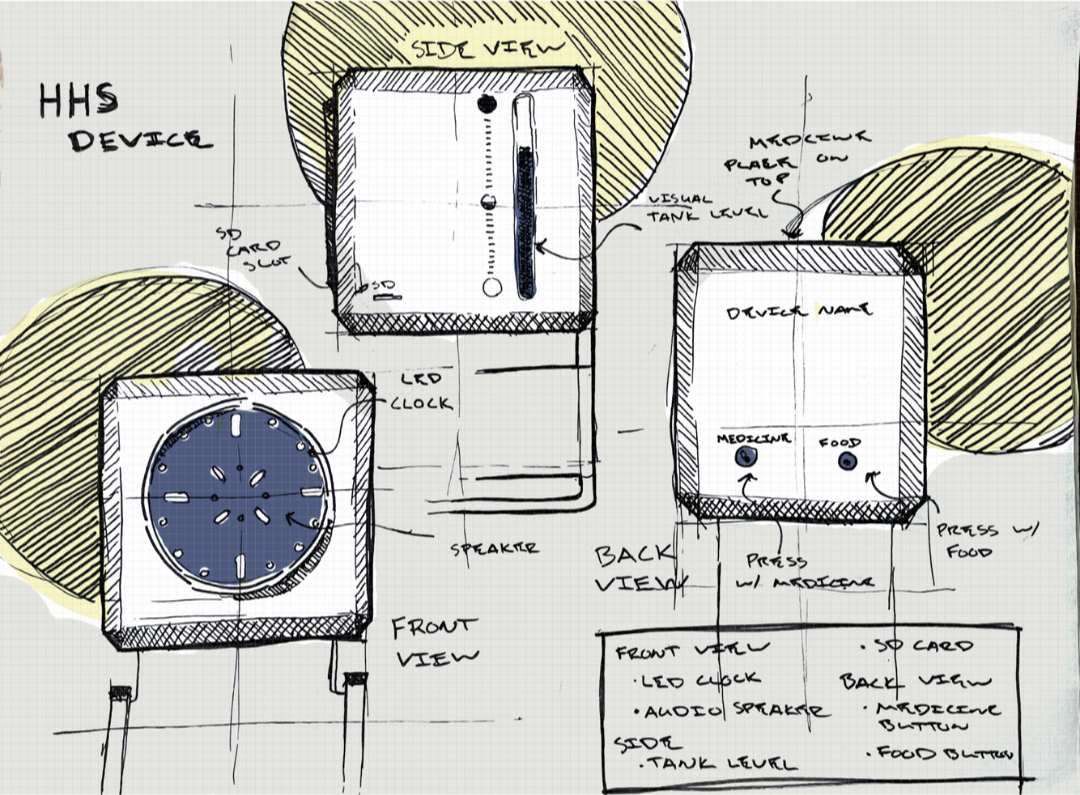
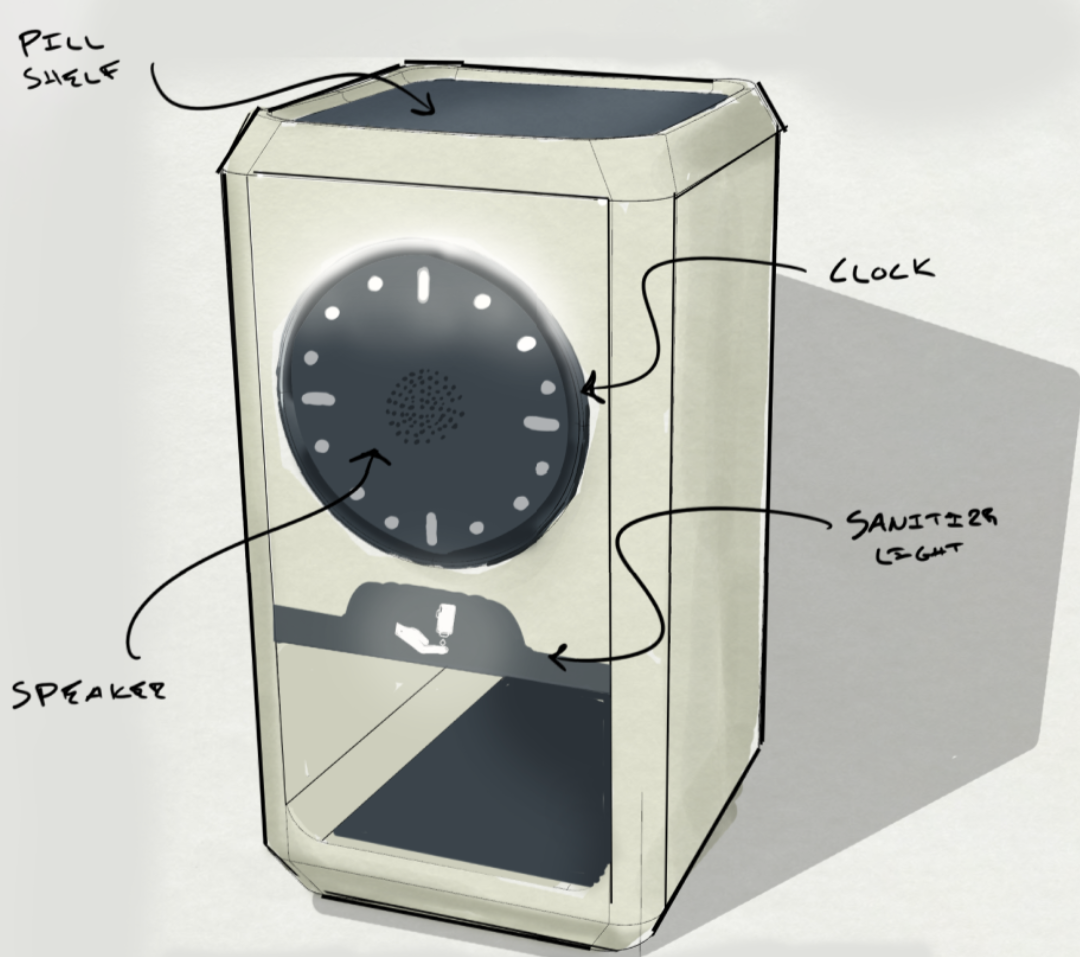
Designing Feedback That Works in Real Healthcare Environments
Auditory Cues: Making Sound Meaningful
Hospitals are saturated with noise—from alarms and intercoms to conversations and machinery. In this environment, generic beeps and tones are easy to miss or ignore, and worse, can trigger anxiety or PTSD in patients and staff alike.
We explored the neuroscience of auditory triggers, especially in trauma-informed environments. PTSD-related sensitivities can include:
Hyperacusis (sound sensitivity)
Tinnitus and phantom sounds
Startle response to normal noises (e.g. water, laughter)
The Solution
We developed a voice-based auditory feedback system, offering clear, calm instructions delivered in a female voice, which proved most effective in conveying urgency without stress. This solution:
Cut through background noise without startling users
Increased user trust and response rate
Respected psychological safety for both patients and providers
"It felt like a person was guiding me—not a machine yelling at me."
— Nurse, Urban ICU
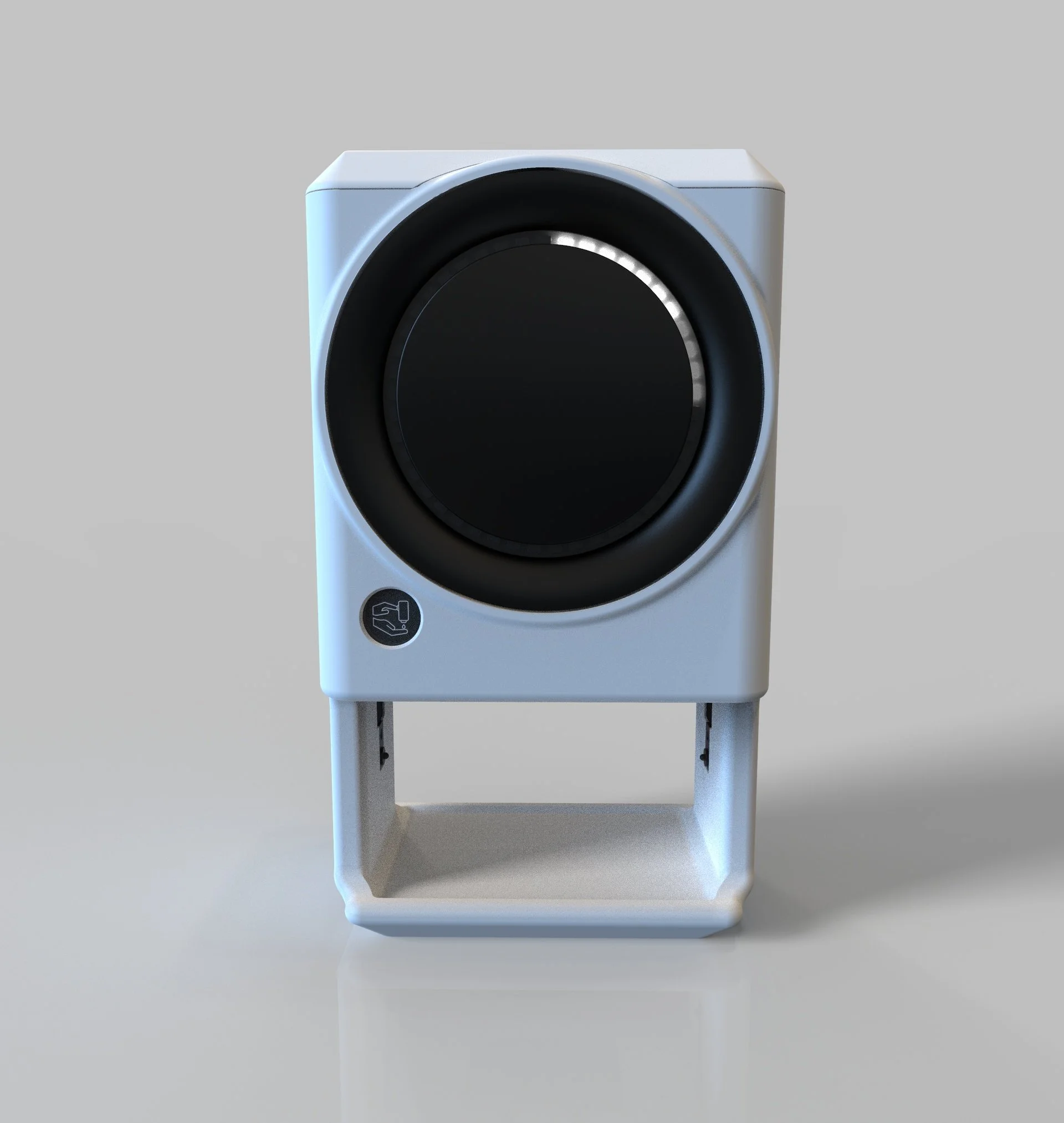
Visual Cues That Guide, Not Distract
The Visual Design Consideration
In emergency rooms and surgical suites, visual noise is constant. Multiple machines compete for attention, and poorly designed feedback can be confusing—or even dangerous for those with photosensitive epilepsy or anxiety disorders.
We collaborated with healthcare staff to map the light environment in real clinical spaces. This informed our approach to visual signaling in high-pressure conditions.
The Solution
A circular RGB LED ring offered optimal clarity, flexibility, and safety. It allowed us to:
Guide users with timed color transitions
Trigger non-invasive prompts that evoke calm urgency
Avoid flashing or strobing effects known to provoke neurological responses
The LED ring enhanced both compliance and engagement—even during high-stress situations—without contributing to alarm fatigue.
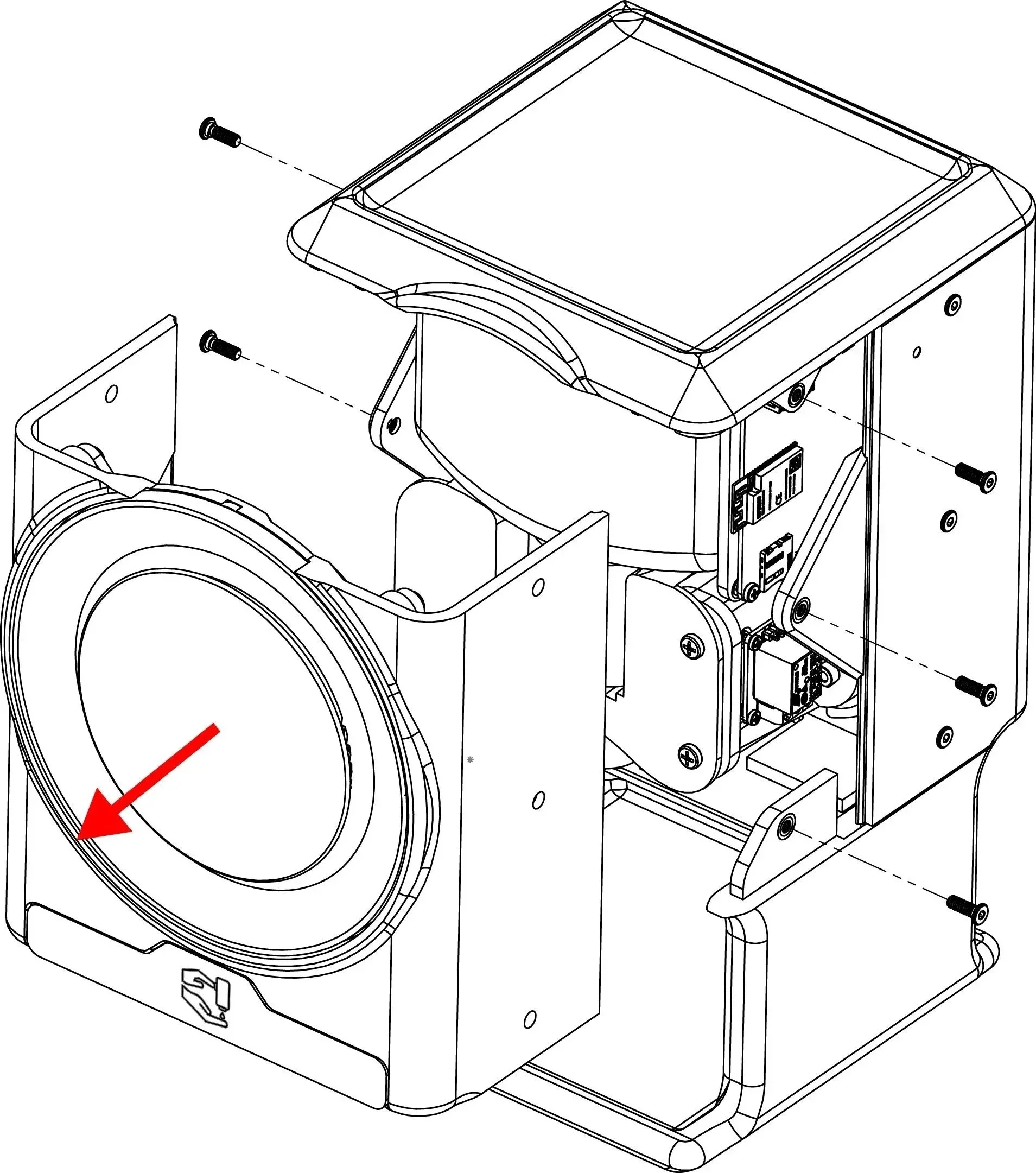
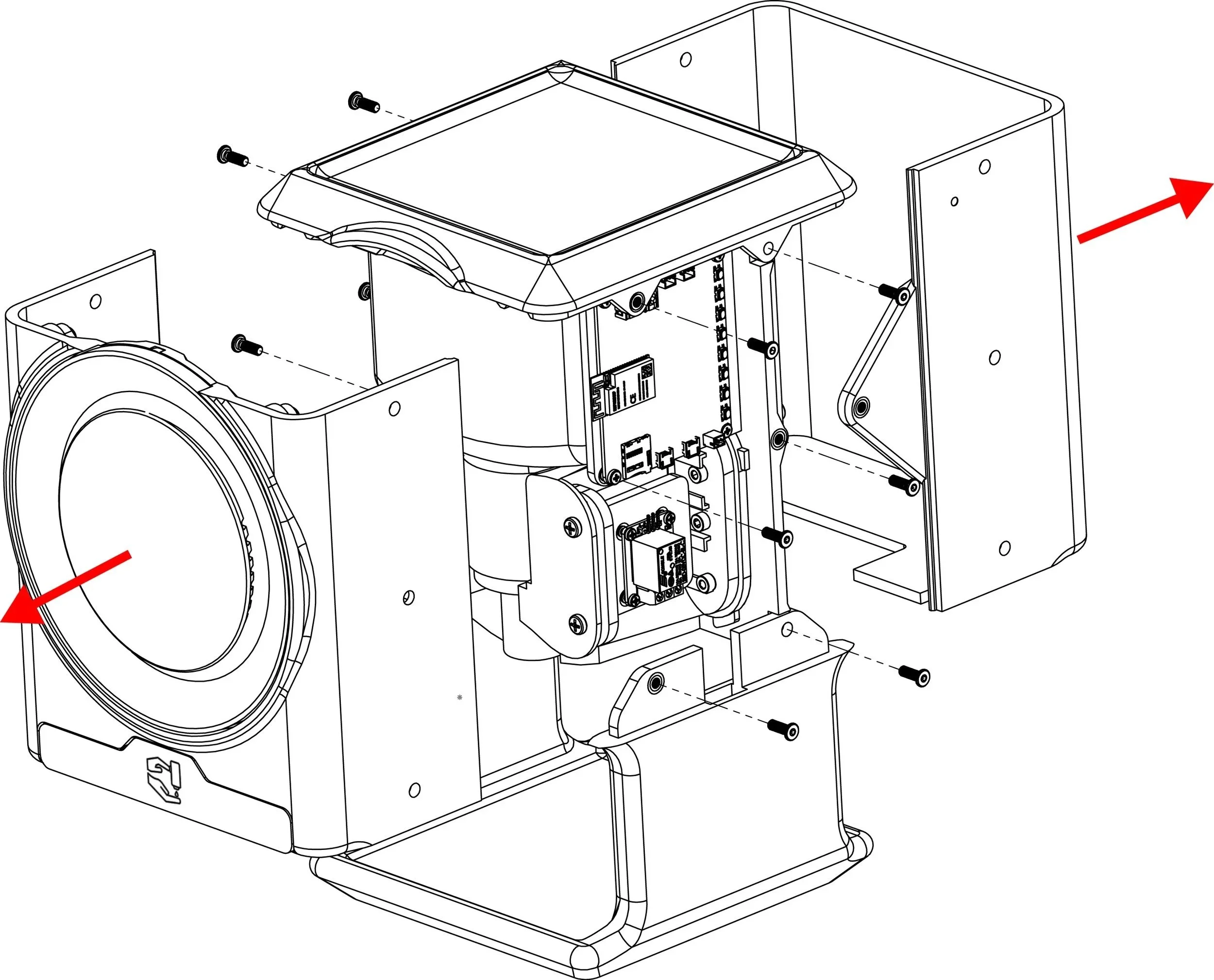
Prototyping and Deployment: From Concept to Clinic
With both visual and auditory cues finalized, we transitioned into rapid prototyping, bridging design intent with technical execution. This phase prioritized real-world integration, risk mitigation, and compliance with global medical device standards.
Starting with the End in Mind
Before CAD, solder, or software, we clarified:
The FDA classification the device would fall under
The sterilization guidelines it would need to meet
Ingress protection (IP) requirements, due to exposed electronics and rechargeable components
These factors drove our material choices, potting strategies, and system architecture—ensuring that regulatory, safety, and usability requirements were baked in from the start.
Real-World Engineering
High-fidelity prototypes tested under clinical lighting and noise conditions
RTOS integration ensured low-latency, real-time interactions
Web dashboard updates enabled improved data collection and user training
Compliant by Design
Regulatory compliance wasn’t retrofitted—it was foundational. From Day 1, our software and system development followed global medical device standards:
IEC 62304 – Structured the medical software lifecycle, from architecture to V&V
ISO 14971 – Governed our risk management practices, with documented hazard identification, control implementation, and residual risk assessment
Applying these standards from the beginning prevented the “compliance catch-up” trap—a common pitfall where retroactive validation and documentation cause costly delays, rework, and redesigns.
By embedding regulatory alignment early, we maintained speed without sacrificing safety or documentation integrity.
Built to Scale
With functional prototypes validated, we moved to production planning, post-market support, and documentation—ensuring a system that could scale beyond pilot sites.
Sterilization & Handling: ISO 17664 Compliance
We developed robust documentation packages to satisfy ISO 17664, which mandates that manufacturers provide clear instructions for:
Cleaning, disinfection, and sterilization
Material compatibility and maintenance
To meet this standard, we created a dual-path documentation approach:
For manufacturers: Step-by-step pre-shipment handling and assembly guidelines
For healthcare facilities: Clear, user-friendly protocols for post-use reprocessing and daily device maintenance
Deployment & Support Highlights
Partnered with manufacturers for supply chain readiness
Developed refurbishment workflows for cost-effective field support
Integrated training materials directly into the digital dashboard
The Result
By treating hygiene feedback as a system—not just a sensor—we created a device that’s intuitive, evidence-backed, and built to last. Landi’s human-centered approach resulted in:
Improved hand hygiene compliance in both public and private facilities
Higher patient satisfaction scores related to perceived cleanliness and safety
Operational ease-of-use for clinical staff across varied environments
A fully compliant path toward regulatory approval and scalable manufacturing